It Starts With A Specimen
In the realm of medical research, the utilization of human biospecimens and biomaterials embarked on a slow and captivating journey. Diverse cultures once viewed autopsies taboo, some even forbidding dissection altogether, opting instead for cremation or organ preservation. However, throughout history, the brilliance of pioneering minds and relentless advancements have paved the way for the establishment and evolution of biobanks, catalyzing a revolution in medical research.[4]
300 B.C.
Around 300 B.C., the trailblazing physicians Herophilus and Erasistratus of Alexandria initiated the earliest dissections for medical research. This marked the dawn of comprehension in human anatomy, laying the groundwork for future discoveries. The 2nd century A.D. witnessed the esteemed physician Galen conducting autopsies to ascertain the cause of death by scrutinizing affected organs. Subsequently, Renaissance luminaries like Leonardo da Vinci embarked on dissections, meticulously crafting precise portrayals of the human body.[4]
1862
Amidst the Civil War in 1862, the Department of Defense (DoD) birthed the first biorepository within the confines of the Army Medical Museum in Washington, D.C. This momentous event marked a profound leap in preserving human specimens for the pursuit of scientific inquiry.[4]
1906
In 1906, Alois Alzheimer's lost slides were found once again, reaffirming the historic significance of the DoD's biorepository as the pioneer of stored specimens.[4]
Mid-1900s
The mid-1900s witnessed the advent of groundbreaking transplant surgeries, triggering the inception of organ donation programs and the emergence of organ banks. Technological strides in preservation techniques facilitated the burgeoning demand for viable organs.[3]
1912
In the 19th century, biologists reshaped their perception, envisioning their work as a comprehensive study of the entire organism. This shift in perspective ushered in the revolutionary cell theory, posing cells as the fundamental building blocks of all living tissue. A 1912 experiment revealed that under the right conditions, cells could be kept alive indefinitely, propelling human tissue research to unexplored heights.[4]
1951
In 1951 patient Henrietta Lacks underwent surgery at Johns Hopkins Hospital, enabling the acquisition of the first cancer cell line, christened HeLa, without her consent. This trailblazing achievement birthed the first immortalized cancer cell line, laying the foundation of cell line biobanking.[1]
1970s-1980s
The success of the HeLa cell line spurred the stabilization and utilization of immortalized cell lines for medical research. This led to the establishment of a multitude of continuous cancer cell lines in the 1970s and 1980s, signifying an era of remarkable progress.[1]
1982
In 1982, the AIDS Specimen Bank was established at the University of California, San Francisco (UCSF), as an AIDS intervention strategy. This pioneering initiative greatly impacted the evolution of biobanking.[1]
1990s-2000s
A remarkable surge in biobanks took place, with 40% of European biobanks being established in the 1990s and an additional 37% of biobanks established in the 2000s.[1]
1996
In 1996 the term "biobank" first appeared in scientific literature, underscoring the growing recognition of these repositories.[4, 5]
1998
1998 was marked by a pivotal event when the Icelandic Parliament passed the Act on Health Sector Database, laying the groundwork for a national biobank in Iceland.[8]
1999
In 1999, the United States National Bioethics Advisory Commission issued a report, outlining policy recommendations concerning the ethical handling of human biological specimens, ushering in a new era of conscientious practice[4, 7]
1999-2000
In 1999, the International Society for Biological and Environmental Repositories (ISBER) was established, with its inaugural meeting held in 2000. This momentous event fostered an atmosphere of collaboration and standardized practices within the biobanking community.[4]
2005
First published in 2005, ISBER's Guide to Best Practices for Repositories solidified its reputation as an authoritative beacon guiding biorepositories and biobanks worldwide.[2]
2005
In 2005, the United States National Cancer Institute created a division called the Office of Biorepositories and Biospecimen Research. This aimed to standardize operating procedures and establish a common database for biospecimen collections.[4, 8]
2006
In 2006 The Council of The European Union adopted a groundbreaking policy, addressing the challenges specific to biobanks, ushering in a new era of regulatory framework.[4, 8]
2008
In 2008 United States researchers stored 270 million specimens in biobanks, along with a new sample collection rate of 20 million samples annually.[8]
2009
In 2009, Time Magazine listed biobanks as one of their "10 Ideas Changing the World Right Now" acknowledging their impact on medical research.[4, 5]
2011
In 2011 the European Biobanking and BioMolecular Resources Research Infrastructure (BBMRI) established the European Research Infrastructure Consortium (ERIC), after a preparatory phase project, setting the framework for an era of collaborative biobanking.[1]
2012
In 2012 the US National Cancer Human Biobank debuted its first-ever Standard Operating Procedures (SOPs) for biobanks, paving the path for consistent and reliable sample collection and data interpretation.[1, 6]
2013
In 2013 the Declaration of Helsinki underwent its last revision, serving as a milestone in the preservation of human rights as it presented a comprehensive set of guidelines for medical research involving human subjects.[1]
2014
In 2014 The ISO/Technical Committee (TC) 2761/Working Group (WG) 2 Biobanks and Bioresources was formed to establish a comprehensive set of international standards for biobanking.[1]
2018
In 2018 the introduction of the "Biobanking—General Requirements for Biobanking" (ISO 20387:2018) guideline allowed for the reproducibility and comparability of scientific research results through standardized biobanking practices.[1]
2018
In 2018, the General Data Protection Regulation (GDPR-2018) provided important indications about Informed Consent (IC) setting forth a legal framework for the collection and processing of personal information.[1]
2019
In May 2019 the Fourth Edition of ISBER Best Practices (2018) launched the LN2 addendum.[9]
We’ve come a long way since the first instances of human tissue collection. In the past, due to limited collaboration, budget and resource constraints, scientists collected biospecimens in isolation, determining best practices on their own, leading to inconsistent processing, hand recorded data and lower-quality samples which often were used only once. These challenges have been replaced with standardized procedures, and the advancements of the biobanks of today playing an indispensable role in medical research, rendering invaluable resources to scientists across the globe.
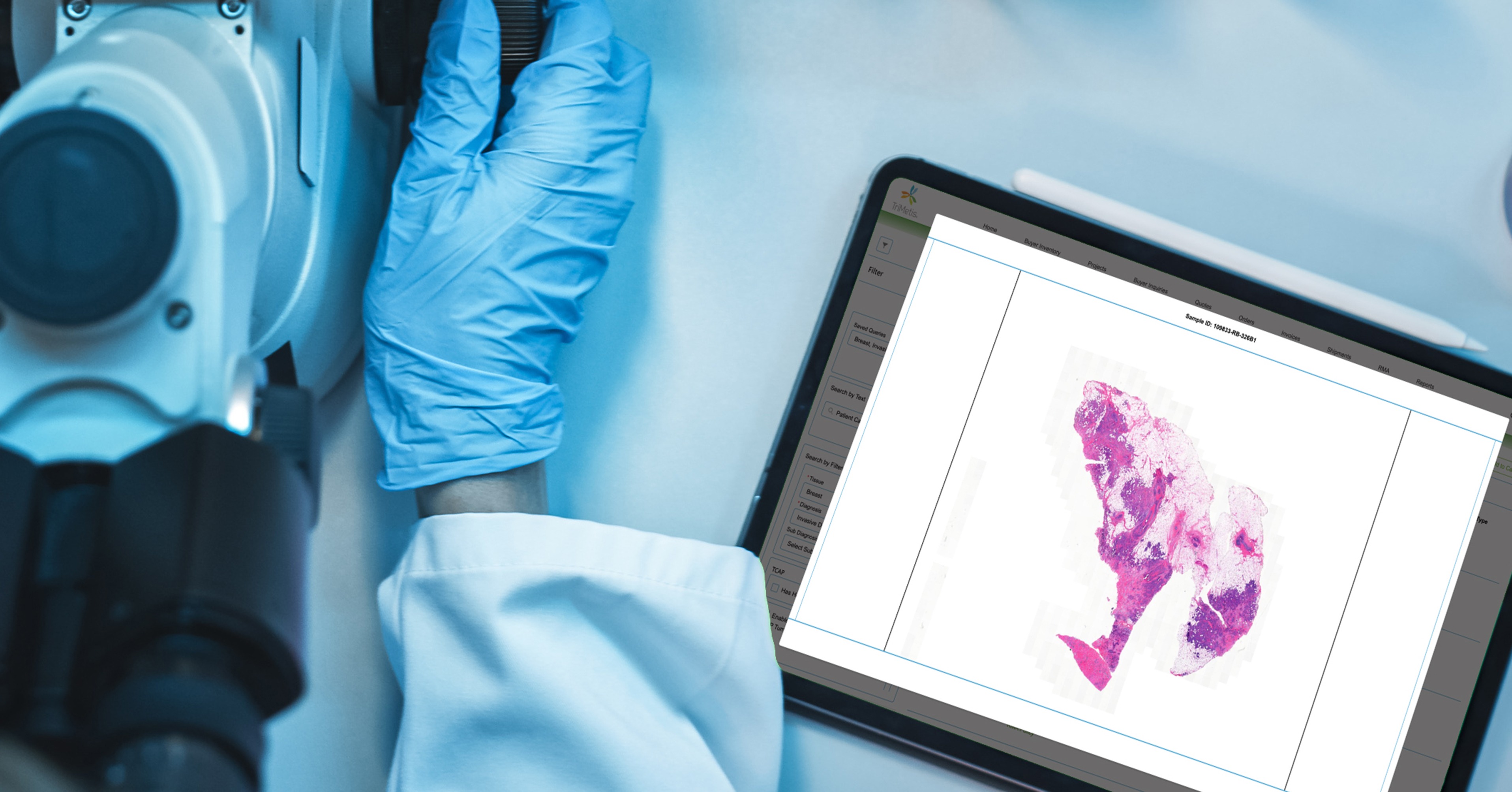
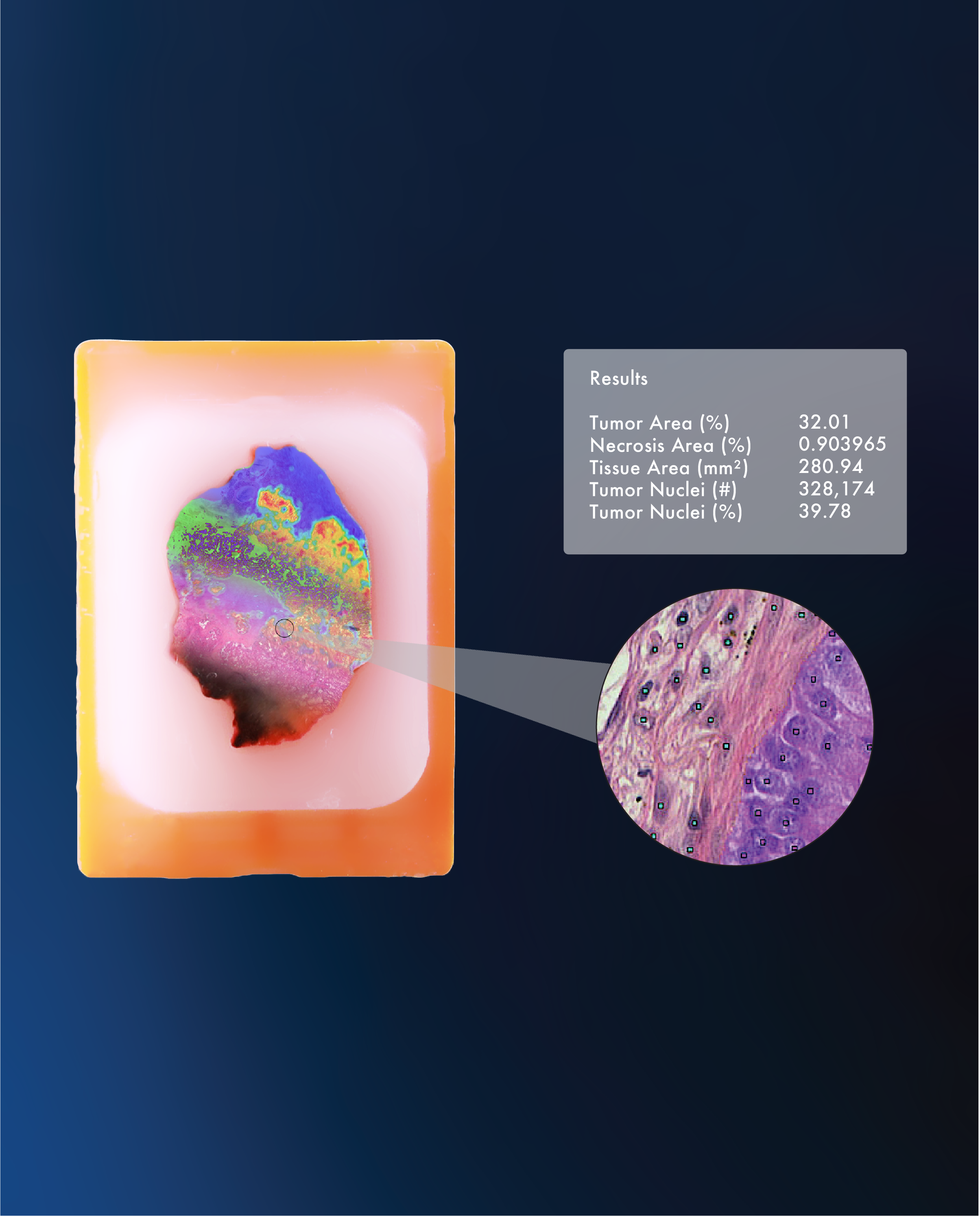
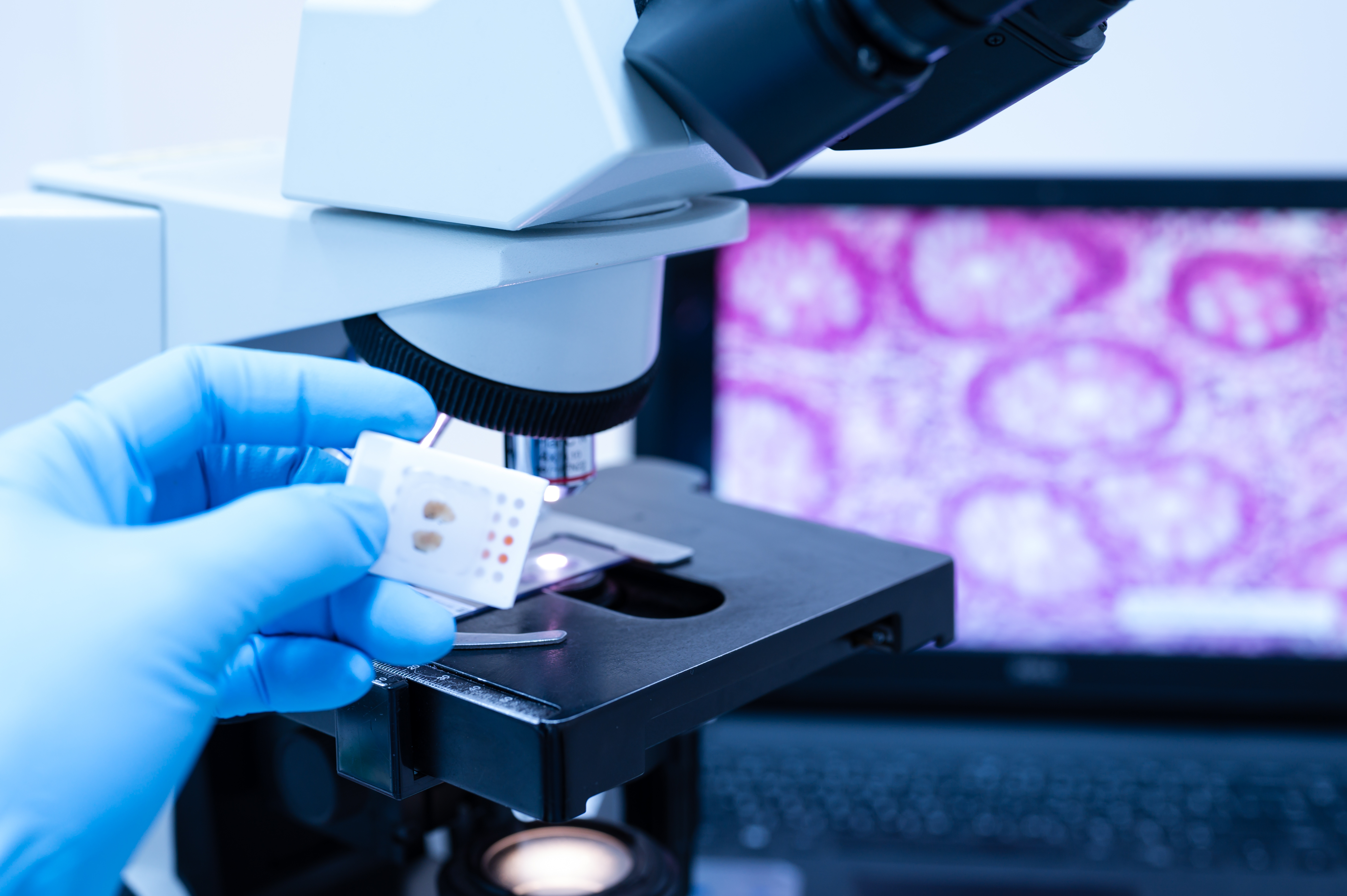
At TriMetis, we’ve leveraged robotic automation, AI-powered quality control, as well as the streamlined digital and physical storage and organization of biospecimens, H&E images, and slides to drastically reduce human handling, increase productivity and tackle the challenges of today head on. We are committed to delivering tailor-made solutions to meet the evolving needs of researchers and the medical community. Contact us to learn more about our custom solution and unlock the boundless potential of biobanking.[3, 4, 5]
Works Cited:
[1] Biobanking in Health Care: Evolution and Future Directions by NIH
[2] The History and Function of Biobanks by LabTag
[3] Handbook of Human Tissue Sources by RAND
[4] Basic Principles of Biobanking: from Biological Samples to Precision Medicine for Patients by Springer Link
[5] Biobanking in Health Care: Evolution and Future Directions by BMC
[6] NIH Standard Operating Procedures (SOPs)
[7] The Past, Present, and Future of Biobanks by Open Specimen
[8] Ethical, Legal, and Social Implications of Biobanks for Genetics Research by Science Direct
[9] ISBER's Best Practices for Repositories